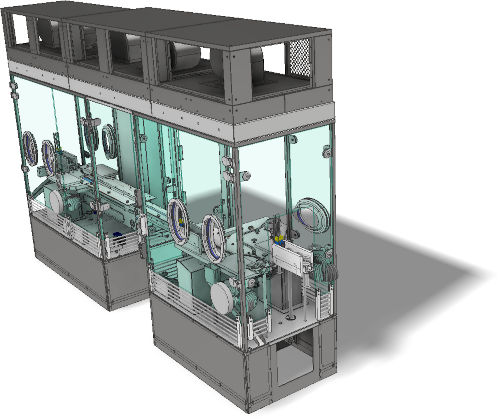
RABS : Restricted Access Barrier System
AVN, your reliable partner in the study and design of RABS (barrier technology) for the compliance of your production tools.
Annex 1 of Good Manufacturing Practices (EU GMP) defines the rules for the manufacture of sterile medicinal products and after several years of comments and revisions, a new version was officially published in August 2022.
It imposes more stringent requirements to reduce the risk of microbial, particulate and pyrogenic contamination to a minimum.
This update will, of course, lead to many changes for the sterile drug production industries. AVN makes all of its experience available to enable quick and efficient compliance of production tools.
AVN integrates all mechanical and automation skills within its design office and ensures the manufacture and assembly in its own
workshop. It is thanks to this control over the complete chain of design and
production that AVN is able to respond with confidence to the different needs.
RABS - TECHNICAL DETAILS
- Open or Closed RABS
- Active (with integrated laminar air flow (LAF)) or Passive (LAF integrated into the ceiling))
- Adaptable and customizable
- Ergonomics validated on real size model (in workshop or on site)
- Control by HMI or Panel PC
- Interlocking
- Traceability (audit trail, CFR21 part11)
